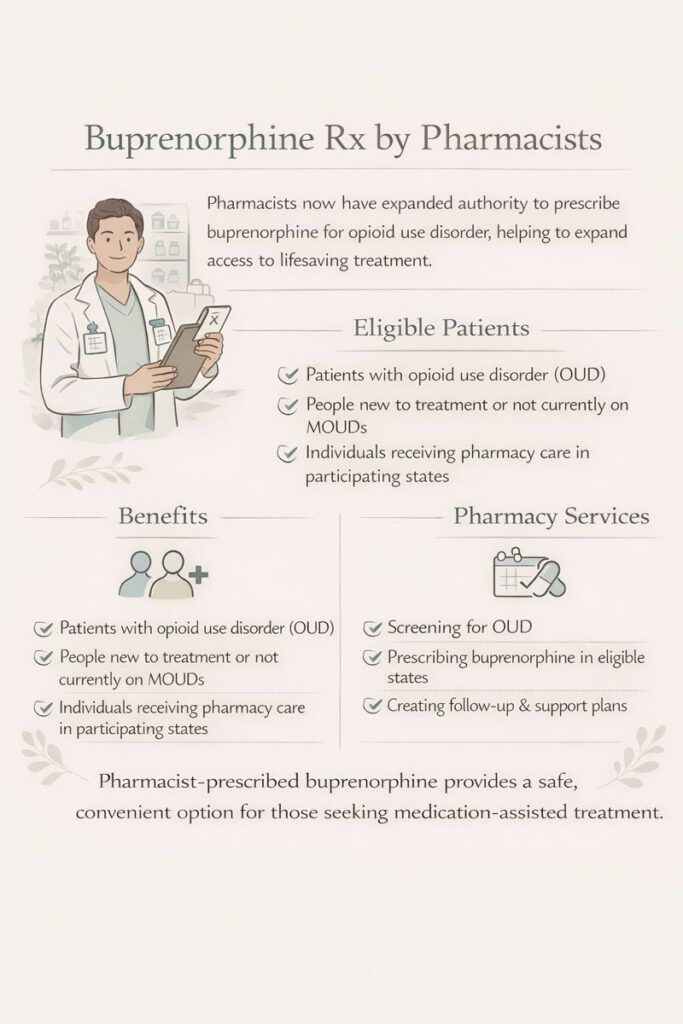
Allowing pharmacists to prescribe buprenorphine for opioid use disorder improves access to treatment, especially in underserved areas. Benefits include convenience and reduced stigma, while challenges involve care coordination and privacy concerns. Ethical issues focus on informed consent and quality of care. Pharmacists must have a valid DEA registration, complete the required training, and comply with state laws to qualify for prescribing.
Pharmacists Can Now Prescribe Buprenorphine: A Game-Changer for Opioid Use Disorder Treatment
Pharmacists are now allowed to prescribe buprenorphine in specific contexts to expand access to treatment for opioid use disorder (OUD)—a response to the ongoing opioid crisis and shortages in addiction care providers. Here’s why this shift is happening:
1. Expand Access to Treatment
- Many areas lack physicians or nurse practitioners trained or willing to prescribe buprenorphine, especially in rural and underserved communities.
- Pharmacists are highly accessible—often the most reachable healthcare providers in a community—and can help fill this gap.
2. Regulatory Changes
- In 2023, the X-waiver requirement was eliminated under the Mainstreaming Addiction Treatment (MAT) Act, making it easier for more professionals—including pharmacists in some states—to prescribe buprenorphine.
- Some states have expanded the scope of practice laws to allow pharmacists to initiate or manage buprenorphine therapy under collaborative agreements or state-specific protocols.
3. Pharmacist Expertise
- Pharmacists are medication experts trained in dosing, side effects, and drug interactions, which is critical in managing buprenorphine therapy safely and effectively.
- Many pharmacists already manage chronic conditions (like hypertension or diabetes), and buprenorphine treatment fits into this expanding clinical role.
4. Reducing Barriers and Stigma
- Patients may feel more comfortable discussing substance use with a pharmacist than with a traditional doctor due to stigma or lack of trust.
- Pharmacist-prescribed buprenorphine can reduce wait times, transportation issues, and the need for multiple appointments.
5. Positive Public Health Impact
- Increasing access to buprenorphine through pharmacies can reduce overdose deaths, improve treatment retention, and destigmatize medication-assisted treatment (MAT).
In summary, pharmacists prescribing buprenorphine reflect a strategic effort to combat the opioid crisis by improving access, reducing provider shortages, and leveraging pharmacists’ expertise. As states update laws and collaborate with healthcare systems, pharmacists are becoming an essential part of addiction care delivery.

Challenges and Considerations When Pharmacists Prescribe Buprenorphine for OUD
While allowing pharmacists to prescribe buprenorphine can expand access to treatment for opioid use disorder (OUD), several potential challenges must be considered:
1. Scope of Training and Experience
- Pharmacists are medication experts but may lack training in addiction counseling, psychiatric assessment, or trauma-informed care.
- Treating OUD often requires holistic approaches, including behavioral health services, which pharmacists may not provide independently.
2. Fragmented Care
- Prescribing outside a coordinated care team can result in disconnected or incomplete treatment.
- Without integrated support, risks such as relapse or untreated co-occurring conditions (e.g., depression, anxiety) may increase.
3. Legal and Regulatory Limitations
- Not all states allow pharmacists to prescribe buprenorphine, and regulations vary widely, creating confusion for providers and patients.
- Pharmacists may face legal liability concerns or lack clarity on best practices without clear guidelines.
4. Workflow and Staffing Issues
- Pharmacies are often busy, with limited private space and staff support.
- Managing OUD treatment requires time-intensive monitoring, which can strain workflows and affect care quality.
5. Risk of Stigma and Discrimination
- Some patients may face judgment or stigma in retail pharmacy settings.
- Not all pharmacists may feel comfortable prescribing buprenorphine without organizational support or addiction-specific training.
6. Limited Follow-Up and Monitoring
- Pharmacists may lack the infrastructure for long-term follow-up, adherence monitoring, or detecting misuse/diversion.
- Without regular drug screens, psychosocial evaluations, or care coordination, some risks may remain unaddressed.
In summary, while pharmacist-prescribed buprenorphine improves access, challenges like fragmented care, limited behavioral health support, regulatory gaps, workflow constraints, and stigma must be addressed to ensure safe, effective, and ethical treatment delivery.
Ethical Considerations for Pharmacists Prescribing Buprenorphine
Using pharmacists to prescribe buprenorphine for opioid use disorder (OUD) raises several ethical dilemmas that require careful consideration:
1. Competence and Scope of Practice
- Pharmacists are medication experts but may lack training in addiction counseling, mental health assessment, and psychosocial care.
- Ethical question: Is it appropriate to expand prescribing rights without ensuring pharmacists can address patients’ full needs?
2. Patient Privacy and Confidentiality
- Pharmacies are often busy, open environments with limited privacy.
- Protecting sensitive patient information and maintaining confidentiality during consultations is an ethical concern.
3. Informed Consent and Autonomy
- Patients must fully understand the benefits, risks, and alternatives to buprenorphine.
- Time constraints and limited counseling training may compromise patients’ ability to make fully informed decisions.
4. Equity and Access
- Expanding pharmacist prescribing may improve access for some populations but leave others behind, such as rural areas without pharmacy services or communities facing stigma.
- Ethical questions arise about fairness and whether this approach reduces disparities.
5. Quality of Care and Continuity
- Without integration into a broader treatment team, care may become fragmented.
- Ethical concern: Is it right to provide medication without comprehensive behavioral support, which is often critical for recovery?
6. Potential Conflicts of Interest
- Pharmacies operate as retail businesses, which may create tension between commercial interests and patient care priorities.
- Business pressures could ethically conflict with clinical decision-making.
7. Stigma and Professional Responsibility
- Pharmacists must manage their own biases and provide nonjudgmental care.
- Ethical duty requires combating stigma that could affect treatment quality and patient dignity.
In summary, while pharmacist prescribing of buprenorphine can expand treatment access, ethical dilemmas related to competence, privacy, informed consent, equity, and quality of care must be carefully addressed. Ensuring safe, respectful, and effective addiction treatment requires thoughtful policies, training, and integrated care approaches.
Pharmacists and Buprenorphine: What You Need to Know About Prescribing for OUD
Pharmacists can now prescribe buprenorphine for opioid use disorder (OUD) in certain circumstances, following changes in federal law and state regulations. The Mainstreaming Addiction Treatment (MAT) Act, enacted in December 2022, removed the federal requirement for practitioners to obtain a “DATA-waiver” (commonly called the “X-waiver”) to prescribe buprenorphine. This change allows qualified practitioners—including pharmacists—to prescribe buprenorphine for OUD treatment, provided they meet specific qualifications and comply with state laws.
Sources: SAMHSA, DEA, Rural Health Info Center
Requirements for Pharmacists to Prescribe Buprenorphine
- Hold a valid DEA registration
- Pharmacists must have a current DEA registration that includes Schedule III authority, as buprenorphine is classified under this schedule.
- Complete required training
- As of June 27, 2023, all practitioners, including pharmacists, must complete an 8-hour training course on the treatment of OUD as a condition of DEA registration or renewal.
- Exceptions apply to those board-certified in addiction medicine or addiction psychiatry, or practitioners who graduated from medical, dental, PA, or advanced practice nursing programs in the U.S. within five years of June 27, 2023.
- Comply with state laws.
- State regulations vary, and some states may have additional requirements or restrictions on pharmacists prescribing buprenorphine.
- Pharmacists must adhere to their state’s specific laws concerning the prescribing of controlled substances.
Key Considerations
While federal law has expanded pharmacists’ prescribing authority, implementation depends on state-specific regulations and may require additional certifications or collaborative practice agreements. Pharmacists interested in prescribing buprenorphine should consult their state pharmacy board and the DEA to ensure full compliance with all requirements.
Frequently Asked Questions
Here are some common questions:
Question: What is the worst scenario if a pharmacist prescribed Buprenorphine?
Answer: The worst-case scenario if a pharmacist prescribes buprenorphine improperly or without sufficient support could involve serious patient safety, legal, and public health risks. Here’s a detailed breakdown:
1. Patient Safety Risks
- Overdose or adverse effects: Buprenorphine, though safer than full opioids, can cause respiratory depression if misused, especially with alcohol or other sedatives.
- Undetected co-occurring conditions: Patients with untreated mental health disorders, chronic pain, or polysubstance use may worsen if care is fragmented.
- Relapse or diversion: Without proper monitoring, buprenorphine could be diverted or misused, leading to addiction or illegal distribution.
2. Fragmented or Inadequate Care
- Lack of behavioral support: OUD treatment is most effective when combined with counseling, peer support, or therapy. A pharmacist acting alone may miss critical psychosocial factors, reducing treatment success.
- Missed complications: Patients may have other medical issues (hepatitis C, HIV, or cardiac problems) that require monitoring; without integrated care, these could go unnoticed.
3. Legal and Professional Consequences
- Regulatory violations: Prescribing outside DEA or state regulations can result in license loss, fines, or criminal charges.
- Malpractice liability: If a patient is harmed due to inadequate assessment or monitoring, the pharmacist could face lawsuits or disciplinary actions.
4. Public Health Impact
- Diversion to the community: If medications are diverted, others could misuse buprenorphine, creating new substance misuse issues.
- Erosion of trust: Poorly managed prescribing could undermine public confidence in pharmacists as healthcare providers.
Summary
The worst-case scenario combines patient harm (overdose, relapse), legal repercussions for the pharmacist, and broader public health consequences. These risks are why pharmacist prescribing must be accompanied by:
Robust monitoring for adherence and diversion
Proper training in addiction management
Care coordination with behavioral health providers
Strict adherence to DEA and state regulations
Question: What is the liability of the pharmacist when prescribing Buprenorphine?
Answer: When a pharmacist prescribes buprenorphine for opioid use disorder (OUD), their liability can be significant, though it depends on federal and state regulations, the setting, and adherence to clinical standards. Here’s a detailed breakdown:
1. Legal and Regulatory Liability
- Scope of Practice: Pharmacists can only prescribe buprenorphine if their state law and federal regulations allow it (e.g., under collaborative practice agreements, specific pilot programs, or newly expanded federal authority under the MAT Act). Prescribing outside these rules can result in disciplinary action, license suspension, or criminal liability.
- DEA Compliance: Buprenorphine is a Schedule III controlled substance. Pharmacists must comply with DEA regulations regarding prescribing, recordkeeping, and diversion monitoring.
- State Law: Some states require additional certifications or protocols for pharmacists prescribing. Ignoring state rules can lead to malpractice or regulatory sanctions.
2. Professional Liability
- Standard of Care: Pharmacists are expected to adhere to evidence-based guidelines for OUD treatment (e.g., dosing, monitoring, screening for contraindications). Failure to do so may be grounds for malpractice claims if a patient is harmed.
- Documentation: Proper documentation of patient assessment, prescribing rationale, follow-up plans, and patient education is crucial. Poor documentation increases liability risk.
3. Patient Harm Liability
- Overdose or Adverse Effects: If a patient experiences an overdose, adverse reaction, or develops complications due to improper prescribing, the pharmacist could be held civilly liable.
- Diversion Risk: If the medication is diverted to others and harm occurs, the pharmacist could face liability if they failed to implement reasonable safeguards.
4. Collaborative or Shared Liability
- If the pharmacist prescribes under a collaborative practice agreement with a physician or clinic, liability may be shared between the pharmacist and the supervising provider.
- Miscommunication or lack of follow-up can increase risk for all parties.
5. Mitigating Liability
- Follow state and federal regulations meticulously.
- Conduct comprehensive patient assessments (opioid use history, other medications, comorbidities, pregnancy status, risk factors for overdose).
- Provide patient counseling on safe use, adherence, and diversion prevention.
- Maintain thorough documentation of all clinical decisions and follow-up plans.
- Use PDMPs (Prescription Drug Monitoring Programs) to check patient prescription history.
Summary:
Pharmacists prescribing buprenorphine carry legal, professional, and civil liability for patient outcomes. Adherence to regulations, standard-of-care guidelines, proper documentation, and patient monitoring is essential to reduce risk. Missteps—such as prescribing outside authority, failing to screen for contraindications, or failing to prevent diversion—could lead to malpractice claims, regulatory action, or criminal charges.
Question: Who oversees the pharmacist who prescribes Buprenorphine?
Answer: Pharmacists who prescribe buprenorphine are overseen by several layers of authority, depending on the context, state laws, and federal regulations. Here’s a clear breakdown:
1. State Board of Pharmacy
- The primary regulatory authority for pharmacists.
- Ensures that prescribing is within the pharmacist’s scope of practice as defined by state law.
- Can investigate complaints, enforce disciplinary actions, suspend licenses, or impose fines for violations.
2. State Medical or Health Board (if under Collaborative Practice)
- In states where pharmacists prescribe under collaborative practice agreements with physicians or other prescribers:
- The supervising physician or clinic often has clinical oversight.
- The state medical board may also monitor adherence to standards for patient safety and prescribing practices.
3. Federal Oversight
- DEA (Drug Enforcement Administration):
- Monitors the prescribing of Schedule III substances like buprenorphine.
- Requires proper registration and adherence to controlled substance regulations.
- Can impose fines or revoke DEA registration for violations.
- SAMHSA (Substance Abuse and Mental Health Services Administration):
- Provides federal guidelines for OUD treatment.
- Ensures adherence to MAT (Medication-Assisted Treatment) standards.
4. Employer or Institutional Oversight
- Pharmacists in hospitals, clinics, or community pharmacies are also monitored by:
- Pharmacy management
- Clinical governance committees
- Quality assurance programs
- These entities often review prescribing patterns, ensure adherence to protocols, and conduct internal audits.
5. Professional Liability Carriers
- Insurance companies may review prescribing behavior if a claim is filed.
- They can require additional training or impose conditions on coverage.
Summary:
The pharmacist’s prescribing of buprenorphine is overseen by a combination of state pharmacy boards, federal agencies (DEA, SAMHSA), collaborative practice physicians (if applicable), employer/clinical governance, and professional liability carriers. This multi-layered oversight ensures both regulatory compliance and patient safety.
Conclusion
Enabling pharmacists to prescribe buprenorphine offers a promising way to expand treatment access and address the opioid crisis. While this approach offers advantages such as greater convenience and reduced stigma, it also poses challenges related to care coordination, privacy, and ethical considerations. Ensuring pharmacists are properly qualified through DEA registration, training, and adherence to state laws is essential to provide safe, effective, and ethical care. Balancing these factors will be key to successfully integrating pharmacists into addiction treatment.
Video: Pharmacists Can Now PRESCRIBE Life-Changing Medication For Opioid Recovery